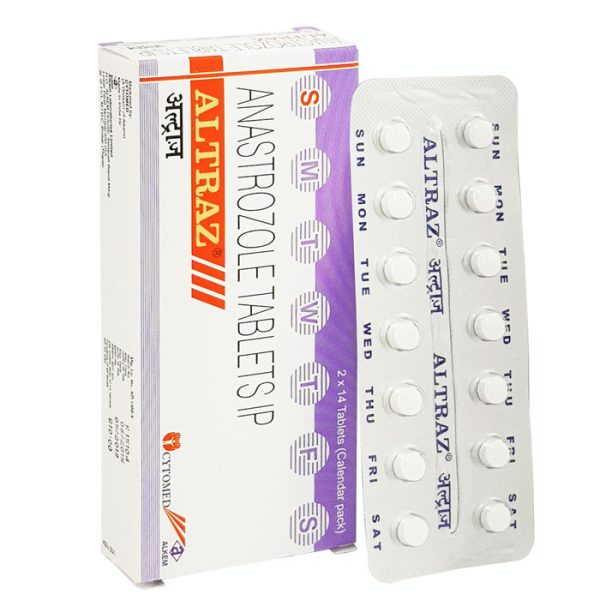
Altraz 1 mg
Altraz 1 mg (Anastrozole) Tablet uses alone or with other treatments, such as surgery or radiation, to treat early breast cancer in postmenopausal women. It operates as the first-line treatment of exceptional breast cancer that has spread within the breast or other body areas.
- Category : Anti Cancer
- Manufacturer : Generic
- Active Substance : Anastrozole
- Customer Rate
- Prescription : Required or Will Provide upon Online Doctor Consultation
Common use
Armidex lowers estrogen levels in postmenopausal women to slow the growth of certain cancer tumors. It works by inhibiting enzyme aromatase and hereby production of estrogen.
Dosage and direction
Follow all instructions of your doctor about treatment and dosing schedule. Do not take more of this medication than it was administered to you. Take once daily.
Precautions
Inform your doctor if you are pregnant or breastfeeding, if you have a severe liver disease, history of stroke of blood clot, heart disease, circulation problems, or if you have not yet completed menopause. If you have such conditions you may need adjustment of your dose. Your treatment with Armidex may continue for five years. This medication may impair your ability to high concentration of attention and thinking or reactions: be especially careful while driving or operating machinery.
Contraindications
Do not use Armidex in premenopausal women, in pregnant and breastfeeding women, severe kidney disorders, moderate and severe liver diseases, hypersensitivity to Armidex or other components of the drug. Preparations which contain estrogens should not be administered with Armidex as they neutralize its pharmacological effect.
Side effects
The most frequent side effects include sings of allergy (hives, skin rash, facial swelling and difficulty breathing) together with sudden headache, confusion, problems with vision, speech, or balance, sudden numbness or weakness, especially on one side of the body, a bone fracture, swollen glands, swelling in your hands or feet. Rare and not so serious adverse reactions may include hot flashes, sore throat, weakness, back pain, bone pain, nausea.
Drug interaction
Inform your doctor if you take tamoxifen or estrogen. Inform your doctor about all prescribed and over -the-counter medications, herbal products, vitamins and food supplements you use.
Missed dose
If you missed a dose take it as soon as you remember. If it is almost time of your next dose just skip it and return to your regular schedule. Never double dose this medication.
Overdose
There is no information about Armidex overdose. If you suspect that you took too much of this medication contact your doctor immediately.
Storage
Store at room temperature between 20-25 C (68-77 F). Store away from moisture, heat, and sunlight. It is not recommended to store in a bathroom and places available for children.
Disclaimer
We provide only general information about medications which does not cover all directions, possible drug integrations, or precautions. Information on the site cannot be used for self-treatment and self-diagnosis. Any specific instructions for a particular patient should be agreed with your health care adviser or doctor in charge of the case. We disclaim reliability of this information and mistakes it could contain. We are not responsible for any direct, indirect, special or other indirect damage as a result of any use of the information on this site and also for consequences of self-treatment.
Altraz 1 mg (Anastrozole) Tablet uses alone or with other treatments, such as surgery or radiation, to treat early breast cancer in postmenopausal women. It operates as the first-line treatment of exceptional breast cancer that has spread within the breast or other body areas.
Reviews
There are no reviews yet.




Reviews
There are no reviews yet.