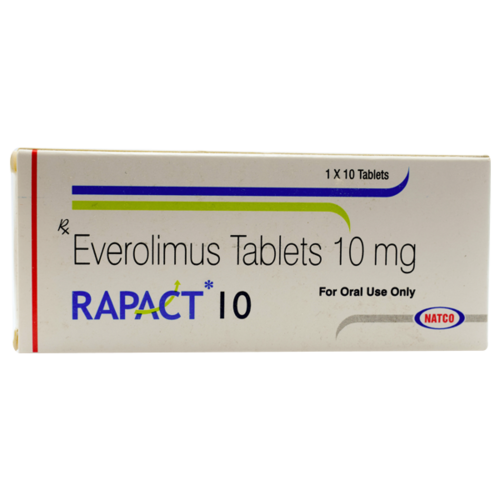
Rapact 10 mg
Rapact 10 mg (Everolimus) Tablet incorporates into a class of meds considered kinase inhibitors that treat malignant growth by preventing disease cells from replicating and slicing off blood supply to the disease cells. Rapact 10mg Tablet diminishes the activity of the invulnerable framework.
- Category : Anti Cancer
- Manufacturer : Generic
- Active Substance : Everolimus
- Customer Rate
- Prescription : Required or Will Provide upon Online Doctor Consultation
PRODUCT INTRODUCTION
Rapact 10mg Tablet must be taken in a dose and duration as per the prescription. It can be taken with or without food but make sure to take it at about the same time every day. An overdose of this medicine may have serious side effects on the body.
The common side effects of this medicine are weakness, sinus inflammation, infection, fever, cough, etc. If any of the side effects persist or bother you, consult the doctor immediately. Inform the doctor immediately if you experience any allergic reactions (rash, shortness of breath, coughing, swelling, etc.). Always seek medical advice if you have increased thirst or increased frequency of urination, suffer from shortness of breath, cough or fever, or need to receive any vaccination after taking this medication.
Before taking this medicine, it is important to consult your doctor if you have any ongoing medication for any underlying disease. Pregnant and breastfeeding women must inform the doctor prior to the initiation of this treatment. During the treatment, your doctor may advise you for some laboratory tests to know the effects of the medicine in the body. It may affect male or female fertility, so consult the doctor if you are planning to have a child.
USES OF RAPACT TABLET
- Treatment of Breast cancer
- Treatment of pancreatic cancer
- Treatment of lung cancer
- Treatment of Kidney Cancer
BENEFITS OF RAPACT TABLET
In Prevention of organ rejection in transplant patients
SIDE EFFECTS OF RAPACT TABLET
Common side effects of Rapact
- Weakness
- Sinus inflammation
- Infection
- Fever
- Cough
- Fatigue
- Stomatitis (Inflammation of the mouth)
- Otitis media (infection of ear)
- Diarrhea
- Upper respiratory tract infection
HOW TO USE RAPACT TABLET
HOW RAPACT TABLET WORKS
Rapact 10 mg (Everolimus) Tablet incorporates into a class of meds considered kinase inhibitors that treat malignant growth by preventing disease cells from replicating and slicing off blood supply to the disease cells. Rapact 10mg Tablet diminishes the activity of the invulnerable framework.
Reviews
There are no reviews yet.




Reviews
There are no reviews yet.